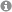Cluster anions of group 14 and 15
Homoatomic anions of the main group elements are popular objects of investigation in fundamental research; they are particularly suitable for studying the linkage patterns of the elements and fundamental aspects of non-polar chemical bonds. However, they are also interesting preparative building blocks in which element clusters are available “naked”, i.e. without substituents. These building blocks can be used to open up new classes of compounds close to elements, including the production of new element modifications.
Synthesis of new Zintl anions
Polyanions of the heavier main group elements, which are often called Zintl ions after the pioneer in this field, can be oxidatively combined from solids to form new, larger particles. One example of this is the synthesis of As???? from two As???. However, new Zintl anions are also formed by the direct reduction of elements or element compounds with solutions of alkali metals in liquid ammonia, such as the ring-shaped Sb??? by the reduction of antimony with lithium or the aromatic P??? by the reaction of diphosphane with caesium.

Fig.1: Dimeric Zintl ion?As???? (left). Sb??? in the ion complex [Li?(NH?)?Sb?]?? (right).
Synthesis of functionalized group 14 and 15 clusters
Another component of our current research is the functionalization of homoatomic polyanions. Transition metal complexes, such as metal carbonyls and NHC complexes, are usually used for this purpose.?



Fig.2:?Different functionalized, homoatomic group 14 and 15 clusters.
NMR spectroscopy in liquid ammoniak
The behavior of the polyanions of group 14 has hardly been researched to date. Spectroscopic investigations in liquid ammonia are therefore of particular interest. Since liquid ammonia can only be handled at low temperatures, special preparation techniques with thick-walled NMR tubes are necessary. Among other things, this made it possible to obtain information about the dynamic behavior of the clusters in solution and the structure of the anions. In addition, the behavior of the protonated species [HSi?]?? in solution could recently be observed.
?
Fig.3: Overview of different NMR signals of silicide clusters in liquid ammonia.
Quantum chemical analysis
The complex chemistry of Zintl clusters can be better understood using quantum chemical methods. By calculating the frontier orbitals of transition metal complexes and functionalized clusters, for example, the coordination of [Au@Pb??]?? as a ligand for Au-NHC complexes can be explained. In addition, by calculating the single point energies of different isomers of Zintl anions, a statement can be made about their relative stability.


Fig.4: Isosurfaces of calculated frontier orbitals (left) and single point energies of different?[Au?Pb??]?? isomeres.