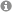Finished Research Topics
Topics with finished research in our workgroup are listed below. To get further information about these topics you can read our papers which you may find in our publication list.
Tinbismuthides
The reactivity of Zintl ions can be changed specifically by substituting single edges in homoatomic clusters by different elements, varying in size, electronegativity and count in valence electrons .
Synthesis of heteroatomic Zintl ions
Heteroatomic Zintl ions can be gained by extraction from mixed valent solid state phases or direct reduction of the elements in liquid ammonia. A variety of tinbismuthides can be prepared via those synthetic pathways. The temperature range of the solvent liquid ammonia between -33 and -78°C also contributes to the stabilization of (energetic) metastable anions like [Sn?Bi?]??.

fig. 1: preparation of varying tinbismuthides.
Group 14 and 15 Chalcogenidometallates
Chalkogenido metallates are attractive building blocks for the rational synthesis of inorganic frameworks by solution reactions. An important model is the rich structural chemistry of silicates, which derives from differently connected SiO? tetrahedra.
Thiosilicate in solution
The solution chemistry of the simplest variation of silicates, in which oxygen is replaced by sulfur, known as thiosilicates from solid state compounds, was completely missing . Through solvatation of SiS? in liquid ammonia? we found (NH?)?[SiS?(NH?)]·2NH? as the first soluble orthothiosilicate (SiS???) derivative.
Chalcogenidoplumbates
Germanates or stannates are known to be soluble in .... In contrast, thio, selenido and tellurido plumbates cannot behandled in these solvents. In liquid ammonia it was possible for the first time to gain both the highly charged original ion PbSe??? and the dimeric compound Pb?Se???.

fig. 1: The monomeric selenido plumbate PbSe??? as it can be found e. g. in the ammoniate Rb?PbSe?·2NH? (left). The dimeric anions Pb?Se??? in cis (mid) or trans conformation (right) which exist in the compounds[Li(NH?)?]?Li?Pb?Se?·5NH? or [Li(NH?)?]?Li?Pb?Se?·4NH?.
Hydrogen Bonding in Liquid Ammonia
In contrast to O?H?O hydrogen bonds in solvate crystals containing water, which are called hydrates, the analogous N?H?N hydrogen bondings in ammoniates have hardly been explored. Through preparation of ammonia-rich crystals these structeres can be classified systematically and compared with hydrates with regard to differences and similarities.
Protonated and deprotonated ammonia clusters - acids and bases in NH?
The strongest N?H?N hydrogen bonds can be observed in interactions to charged species. Both the ammonium ion NH?? as acid in the ammonia system, and amide NH?? as the base in the ammonia system form ammonia rich salts. During our invertigations the structure of protonated ammonia clusters like [NH?(NH?)?]?, [(NH?)?(NH?)??]??, ∞?[NH?(NH?)?(?-NH?)?]? (fig.1) and ∞?[{N??H??}(?-NH?)?]??could be characterised for the first time. Dissolving alkaline metal amides in ammonia leads to recrystallization of ammonia rich compounds like KNH?·2NH? and RbNH?·2/3NH?.

fig. 1: one dimensional complex strand of ∞?[NH?(NH?)?(?-NH?)?]? (the coordination environment of the ammonium ions is highlighted as blue tetrahedra)
Ammoniates in sugars and carboxylic acids
The interactaions of organic species like carboxylic acids, polyoles and carbohydrates with liquid ammonia have also hardly been explored. Potentially ammonia is an interesting alternative to polar solvents containing oxygen. The focus of this topic were solvability studies, structural chemistry of crystallizing ammoniates (fig. 2) and simple reactions with carbohydrates.

fig. 2: hydrogen bonds surrounding a polyole molecule in sorbitole pentaammoniate (left). Crystal structure of ammonium capronate monoammoniate, NH?(C?H??COO)·NH? (right)